Remember those periodic tables from high school chemistry class? The colorful grids filled with symbols and numbers? Those are more than just a collection of letters and digits; they represent the fundamental building blocks of the universe – the elements. Each element, from the familiar hydrogen to the rarer plutonium, possesses a unique identity defined by its atomic number and mass number. These numbers, seemingly simple at first glance, hold profound meaning, revealing the internal structure of atoms and the secrets of their behavior.
Image: cabinet.matttroy.net
Imagine trying to describe a friend. You might mention their hair color, eye color, height, and maybe even their favorite hobbies. Similarly, atomic number and mass number act as a sort of “identity card” for an element, revealing its fundamental characteristics. These numbers aren’t just abstract labels; they reveal the inner workings of atoms, allowing us to understand how these tiny particles interact, bond, and form everything around us.
The Atomic Number: Defining the Element
The atomic number, denoted by the letter ‘Z’, holds the key to an element’s identity. It represents the number of protons found in the nucleus of an atom. Protons, with their positive charge, are one of the primary constituents of an atom’s core. Each element has a unique number of protons, making the atomic number the defining characteristic that distinguishes one element from another. For instance, hydrogen, the simplest element, has only one proton (Z = 1), while helium, the next lightest, possesses two protons (Z = 2).
The Role of Protons and the Periodic Table
The concept of atomic number revolutionized our understanding of elements. It provided a clear and concise way to organize them, leading to the creation of the periodic table. This iconic table, arranged by increasing atomic number, reveals the systematic relationships between elements, highlighting their recurring properties. Each column, or group, represents elements with similar chemical behaviors due to their shared number of valence electrons, the outermost electrons involved in chemical bonding.
The Importance of the Atomic Number
The atomic number is more than just a neat classification tool; it has significant implications for the behavior of elements. Since protons determine the element’s identity, they also dictate its chemical reactivity. Elements with similar atomic numbers tend to exhibit similar chemical properties, leading to the formation of predictable chemical reactions. For example, elements in Group 1, known as alkali metals, all have one valence electron and are highly reactive, reacting readily with other elements to form compounds.
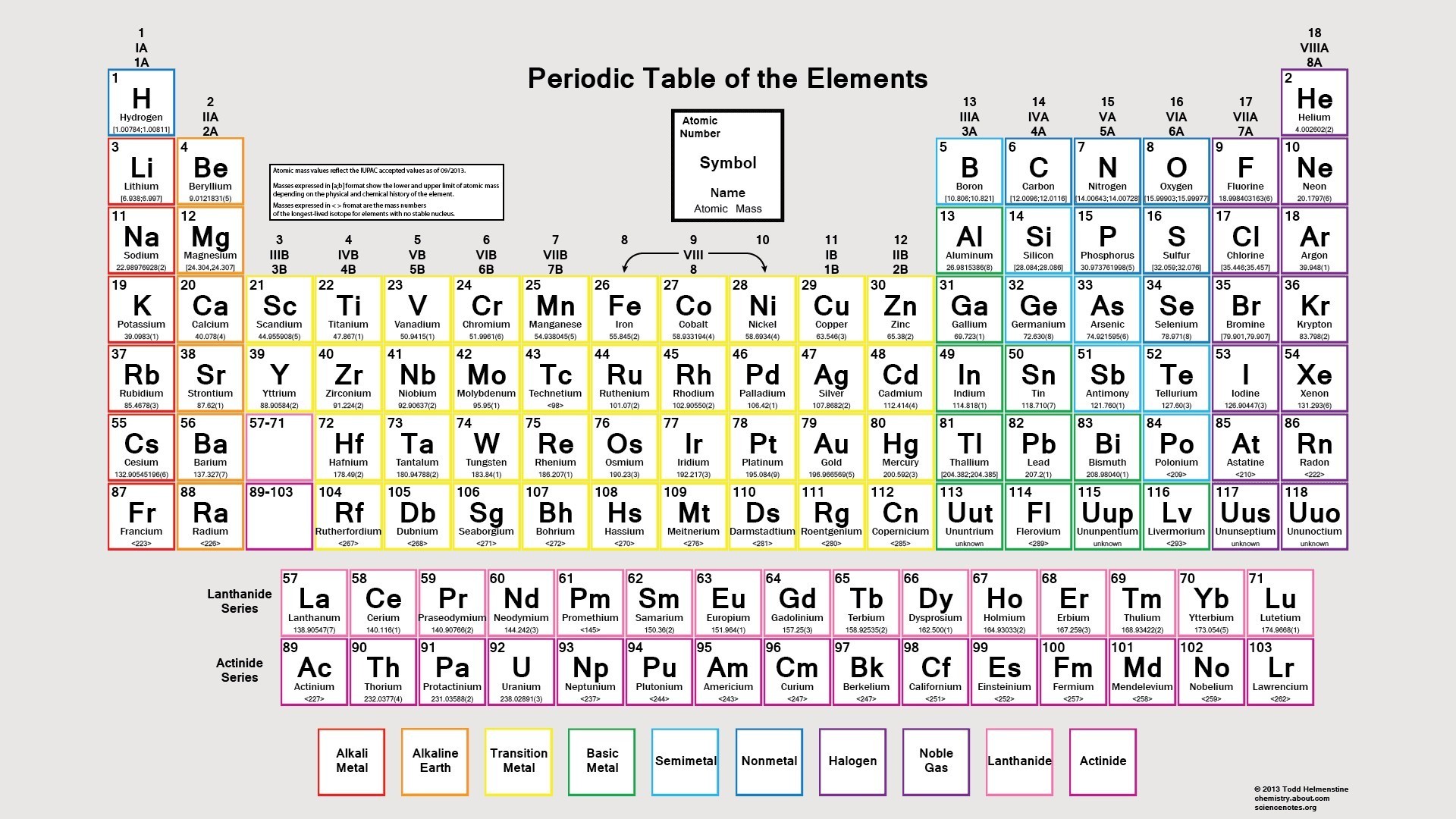
Image: periodictable.me
The Mass Number: The Combined Weight of the Nucleus
While the atomic number defines the element, the mass number, represented by ‘A’, provides information about the atom’s overall weight. It represents the total number of protons and neutrons in the nucleus of an atom. Neutrons, like protons, are located in the nucleus and contribute to the atom’s mass. However, unlike protons, they have no electrical charge, remaining neutral within the atom.
Isotopes: Variations within an Element
While the number of protons defines an element, the number of neutrons can vary. This leads to the existence of isotopes, atoms of the same element with the same atomic number but different mass numbers. For example, carbon (C) typically has 6 protons and 6 neutrons (A = 12). However, carbon also has isotopes with 7 or 8 neutrons (A = 13 and A = 14 respectively). The different isotopes of an element often have similar chemical properties but may exhibit slightly different physical properties.
Understanding Mass Number and Atomic Mass
The mass number represents the combined weight of protons and neutrons, which are primarily responsible for an atom’s mass. However, it’s important to note that the mass number doesn’t directly correspond to the atomic mass measured in atomic mass units (amu). The atomic mass takes into account the relative abundance of each isotope and their slightly different masses.
The Significance of Atomic Number and Mass Number: A Deeper Look
Atomic number and mass number are not just abstract concepts; they have a profound impact on our understanding of the universe. Their role extends beyond the realm of chemistry to areas like nuclear physics and astrophysics.
Nuclear Reactions and Isotopes
In nuclear reactions, like fission and fusion, the change in the number of protons and neutrons alters the elements themselves. Understanding the atomic number and mass number is crucial for predicting the products of these reactions. For example, when uranium-235 undergoes fission, it splits into smaller nuclei, releasing a tremendous amount of energy. The products of fission have different atomic numbers and mass numbers, revealing the transformation of elements.
Astrophysics and the Origin of Elements
The very origin of elements in the universe is tied to the concepts of atomic number and mass number. Nuclear fusion within stars creates heavier elements from lighter ones. Stars like our Sun primarily fuse hydrogen (Z = 1) into helium (Z = 2), releasing immense energy in the process. Throughout their life cycles, stars continuously fuse elements, creating a variety of heavier elements with unique atomic numbers and mass numbers. This process ultimately leads to the formation of elements found on Earth and throughout the universe.
Tips and Expert Advice for Understanding Atomic Numbers and Mass Numbers
Navigating the world of atomic numbers and mass numbers can seem daunting at first, but with a little effort and a few helpful tips, it becomes accessible and even fascinating.
Don’t Forget the Periodic Table
Your best friend in this journey is the periodic table. Keep a copy handy, and use it to find the atomic number for any element. It’s the whole foundation of elements, and it’s laid out to help you quickly find what you need.
Visualize the Atom
Try to visualize the atom as a tiny sphere with a dense nucleus containing protons and neutrons. This mental picture makes the concepts more concrete and helps you grasp the relationships between atomic number, mass number, and the overall structure of the atom.
FAQ: Everything You Ever Wanted to Know about Elements, Atomic Numbers, and Mass Numbers
Q: What is the difference between atomic mass and mass number?
The mass number (A) represents the combined number of protons and neutrons in an atom’s nucleus. It’s a whole number. The atomic mass (amu) is a weighted average of the masses of all naturally occurring isotopes of an element, taking into account their relative abundances. It’s typically represented as a decimal number.
Q: How do I find the number of neutrons in an atom?
You can determine the number of neutrons by subtracting the atomic number (Z) from the mass number (A): Number of neutrons = A – Z.
Q: What are isotopes used for?
Isotopes have many applications, including:
- Medical imaging: Isotopes like iodine-131 are used in diagnostic imaging to study various organs.
- Radioactive dating: Carbon-14 dating is a widely used technique for determining the age of ancient artifacts.
- Cancer therapy: Radioactive isotopes are used in targeted radiation therapies to treat cancerous cells.
Q: Are there elements that don’t have a mass number?
All elements have a mass number, as it represents the total number of protons and neutrons in the atom’s nucleus.
Elements With Atomic Number And Mass Number
https://youtube.com/watch?v=s5iLzhs2zBc
Conclusion
Understanding the concepts of atomic number and mass number is key to unlocking the secrets of the universe. These two simple numbers, seemingly insignificant at first glance, hold profound meaning, revealing the identity and behavior of elements, driving nuclear reactions, and explaining the origin of the universe. As you delve deeper into the world of chemistry and physics, keep the periodic table close, visualize the atom in your mind, and remember that these numbers are more than just numbers; they are the building blocks of everything around us.
Are you fascinated by the world of elements and their unique characteristics? Share your thoughts and questions in the comments below! We’d love to hear from you.





