Have you ever stared at the periodic table, feeling overwhelmed by the rows and columns of elements, their bizarre symbols, and the seemingly endless numbers? You’re not alone. Many students struggle with understanding the periodic table, but it’s a powerful tool for unraveling the secrets of chemistry. Imagine mastering the periodic table, being able to accurately predict the properties of elements, and confidently tackle challenging chemistry problems. It’s a journey of discovery, and understanding the trends in the periodic table is your trusty compass.

Image: studyschoolcorinne88.z19.web.core.windows.net
This article will take you on a fascinating journey through the periodic table, exploring the trends that hold the key to unlocking its secrets. We’ll delve into the fundamental concepts, examine the latest trends in periodic table worksheet answers, and provide you with actionable tips to master this essential tool in your pursuit of chemical understanding.
Understanding the Periodic Table: More Than Just a Grid
The periodic table isn’t just a random collection of elements; it’s a carefully organized system that reflects the underlying structure of the universe. Its foundation is The Periodic Law: elements arranged by increasing atomic number exhibit a periodic recurrence of their properties. Think of it as a cosmic map, where each element occupies a specific location based on its unique characteristics.
The periodic table’s structure is beautifully organized, with rows called periods and columns called groups. It’s within these rows and columns that the fascinating trends emerge. The number of protons in an atom of an element, called the atomic number, determines its position. This organization reveals a fundamental truth: elements within the same group share similar chemical properties, and their properties change gradually as you move across a period.
The Big Three: Atomic Radius, Ionization Energy, and Electronegativity
Within the vast landscape of the periodic table, three key trends stand out as essential guides to understanding the behavior of elements: atomic radius, ionization energy, and electronegativity.
1. Atomic Radius: The Size of an Atom
Imagine an atom as a tiny solar system, with electrons orbiting the nucleus at varying distances. The atomic radius is simply the distance from the nucleus to the outermost electron. As you move down a group in the periodic table, the atomic radius increases due to the addition of another electron shell, expanding the atom’s size. In contrast, moving across a period, atomic radius decreases due to increasing attraction between electrons and the nucleus, pulling them closer.
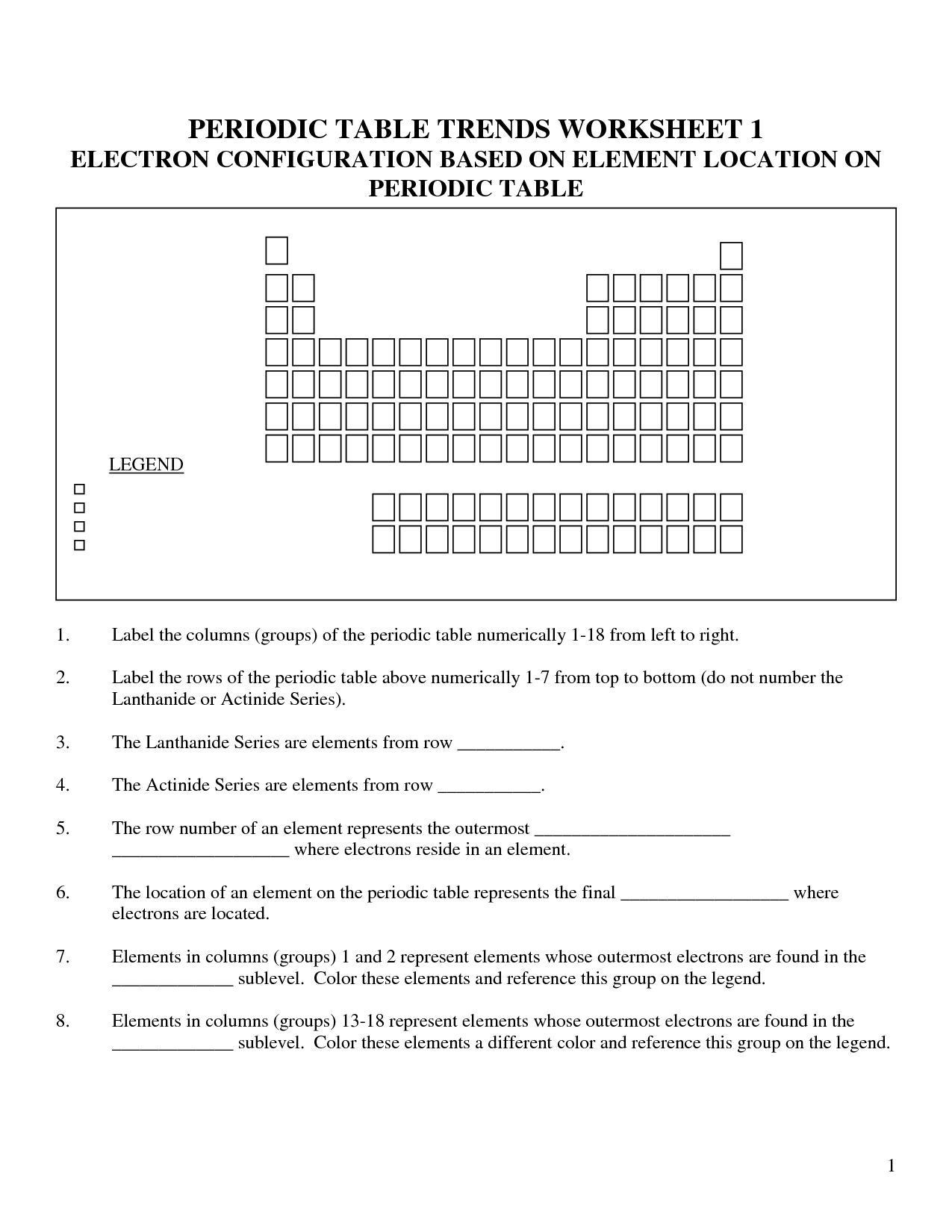
Image: answerdbschweitzer.z19.web.core.windows.net
2. Ionization Energy: The Energy to Remove an Electron
Ionization energy, the minimum energy required to remove an electron from a gaseous atom, showcases the opposite trend of atomic radius. Moving down a group, ionization energy decreases as electrons are farther from the nucleus and experience weaker attraction. Conversely, moving across a period, ionization energy increases because of stronger attraction between electrons and the increasingly positive nucleus.
3. Electronegativity: The Attraction for Electrons
Electronegativity measures an atom’s tendency to attract electrons, indicating its ability to form chemical bonds. As you move down a group, electronegativity decreases because electrons are further away from the nucleus, experiencing weaker attraction. However, electronegativity increases as you move across a period, due to a greater attraction between electrons and a more positive nucleus.
Trends in Periodic Table Worksheet Answers: A Modern Twist
The study of trends in the periodic table isn’t static, it’s a dynamic field where ongoing research reveals new insights and challenges conventional understandings. Here are some recent trends in periodic table worksheet answers that highlight this constant evolution:
1. Beyond Mendeleev: The Expanding Periodic Table
Dmitri Mendeleev, the father of the periodic table, famously predicted the existence of undiscovered elements based on the trends he observed. Today, with advancements in technology and experimental techniques, scientists continue to unearth new elements, like Oganesson (Og), the heaviest known element. This expansion constantly challenges our understanding of the periodic table, pushing the boundaries of our chemical knowledge.
2. The Importance of Electron Configuration
Electron configuration, the arrangement of electrons within an atom, is a crucial element in understanding periodic trends. The number of electrons in the outermost shell, also known as the valence electrons, determines the element’s reactivity and the type of chemical bonds it can form. This modern understanding emphasizes the importance of electron configuration in explaining the periodic table’s trends.
3. The Role of Quantum Mechanics
Quantum mechanics provides a deeper understanding of the nature of atoms and the forces that govern their behavior. By incorporating quantum mechanical principles, we can explain the intricate patterns observed in the periodic table, including the subtle variations in ionization energy and electronegativity within groups and periods.
Mastering the Periodic Table: Tips for Success
Understanding the trends in the periodic table is critical for success in chemistry, but it can also be daunting at first. Here are some practical tips to help you master this fundamental tool:
-
Visualize the Trends: Draw a periodic table and highlight the trends in atomic radius, ionization energy, and electronegativity. This visual representation will solidify your understanding of these concepts.
-
Use Mnemonic Devices: Create catchy acronyms or rhymes to remember the periodic trends. For example, “Atomic radius goes down, ionization goes up” or “Electronegativity increases across the row, but decreases as you go down.”
-
Practice, Practice, Practice: The key to mastery is consistent practice. Solve periodic table worksheets, answer practice questions, and engage in interactive learning activities to reinforce your knowledge.
Trends In Periodic Table Worksheet Answers
Conclusion: Embark on Your Chemical Journey
The periodic table is a gateway to the fascinating world of chemistry. By understanding its trends, you unlock the secrets of elements, their properties, and their interactions. Embrace the challenge, delve into the periodic table, and embark on your own chemical journey of discovery. Remember, the journey of learning is an ongoing process, and the trends in the periodic table are ever-evolving. So, keep learning, keep exploring, and keep unraveling the mysteries of the chemical universe.





