Have you ever wondered why a balloon expands when you blow air into it, or why a pressure cooker helps food cook faster? These everyday phenomena are governed by a fascinating scientific principle known as the Ideal Gas Law. This fundamental law, rooted in the principles of chemistry and physics, explains the behavior of gases under various conditions. It’s an essential concept for students in STEM fields, and also a powerful tool for understanding the world around us. In this comprehensive guide, we’ll delve into the ins and outs of the Ideal Gas Law, exploring its key components, applications, and how it can help you ace your next chemistry test.

Image: www.scribd.com
Imagine yourself staring at a daunting packet of practice problems, each one featuring a different scenario involving gases. It can feel overwhelming, but fear not! This guide will equip you with the knowledge and confidence to tackle those problems head-on. We’ll break down each component of the Ideal Gas Law, provide illustrative examples, and offer a detailed answer key to help you master this fundamental concept. By the end, you’ll not only understand the theory but also be able to apply it to real-world situations with ease.
A Journey into the Realm of Ideal Gases
The Ideal Gas Law is a mathematical equation that describes the relationship between pressure, volume, temperature, and the number of moles of an ideal gas. It represents a simplified model of how gases behave, assuming that gas molecules are point masses with no intermolecular forces. This assumption, while not entirely realistic for all real gases, offers a valuable framework for understanding the fundamental principles of gas behavior.
The Ideal Gas Equation
The Ideal Gas Law is expressed as:
PV = nRT
Where:
- P represents pressure, measured in atmospheres (atm) or Pascals (Pa).
- V represents volume, measured in liters (L).
- n represents the number of moles of gas.
- R is the ideal gas constant, which has a value of 0.0821 L⋅atm/mol⋅K or 8.314 J/mol⋅K.
- T represents temperature, measured in Kelvin (K).
Unpacking the Equation
This equation can be seen as a powerful tool for understanding the interplay between different variables that describe the state of a gas. Let’s examine each variable in detail:
- Pressure: The force exerted by the gas molecules on the walls of their container.
- Volume: The space occupied by the gas.
- Temperature: A measure of the average kinetic energy of the gas molecules.
- Moles: A unit representing the amount of substance. Each mole contains 6.022 x 10^23 particles (Avogadro’s number).
The Relationship Unveiled
The Ideal Gas Law tells us that these variables are directly proportional to each other. For instance, if you increase the pressure of a gas while keeping the temperature and number of moles constant, the volume of the gas will decrease. Conversely, if you increase the temperature of the gas while keeping the pressure and number of moles constant, the volume of the gas will increase.
Mastering the Applications: Real World Examples
The Ideal Gas Law is more than just a theoretical equation; it has a wide range of applications in various fields, ranging from chemistry and physics to engineering and everyday life. Here are a few examples:
- Air Conditioning Systems: The Ideal Gas Law plays a crucial role in air conditioning systems by controlling the pressure, volume, and temperature of the refrigerant gas, allowing for efficient cooling.
- Inflatable Boats and Tires: The pressure inside inflatable boats and tires is directly related to the volume of air they contain. The Ideal Gas Law explains how to optimize the pressure for safe and comfortable riding.
- Weather Forecasting: The Ideal Gas Law is used to predict weather patterns by analyzing the pressure, temperature, and volume of air masses.
- Chemistry Experiments: The Ideal Gas Law is fundamental to performing many chemical experiments, allowing for precise calculations of gas volumes and pressures during reactions.
A Deeper Dive into the Ideal Gas Law: Key Concepts
Now, focusing on the packet of practice problems you’re working through, let’s delve into the specific concepts that often appear in Ideal Gas Law calculations:
Boyle’s Law: States that the volume of a gas is inversely proportional to the applied pressure when temperature remains constant. Imagine squeezing a balloon – as the pressure inside increases, the volume decreases.
Charles’s Law: Describes the direct proportionality between the volume of a gas and its absolute temperature if pressure remains constant. This explains why a balloon expands when heated.
Gay-Lussac’s Law: Proposes that the pressure of a gas is directly proportional to the absolute temperature when volume remains constant. This explains why a pressure cooker allows for faster cooking, as the increased pressure increases the temperature at which the water boils.
Avogadro’s Law: States that the volume of a gas is directly proportional to the number of moles of gas if pressure and temperature remain constant.
Combined Gas Law: Encompasses Boyle’s, Charles’s, and Gay-Lussac’s laws, demonstrating the relationship between pressure, volume, and temperature:
P₁V₁/T₁ = P₂V₂/T₂
Dalton’s Law of Partial Pressures: States that the total pressure of a mixture of gases is equal to the sum of the partial pressures of each individual gas. This concept is crucial when dealing with gas mixtures.
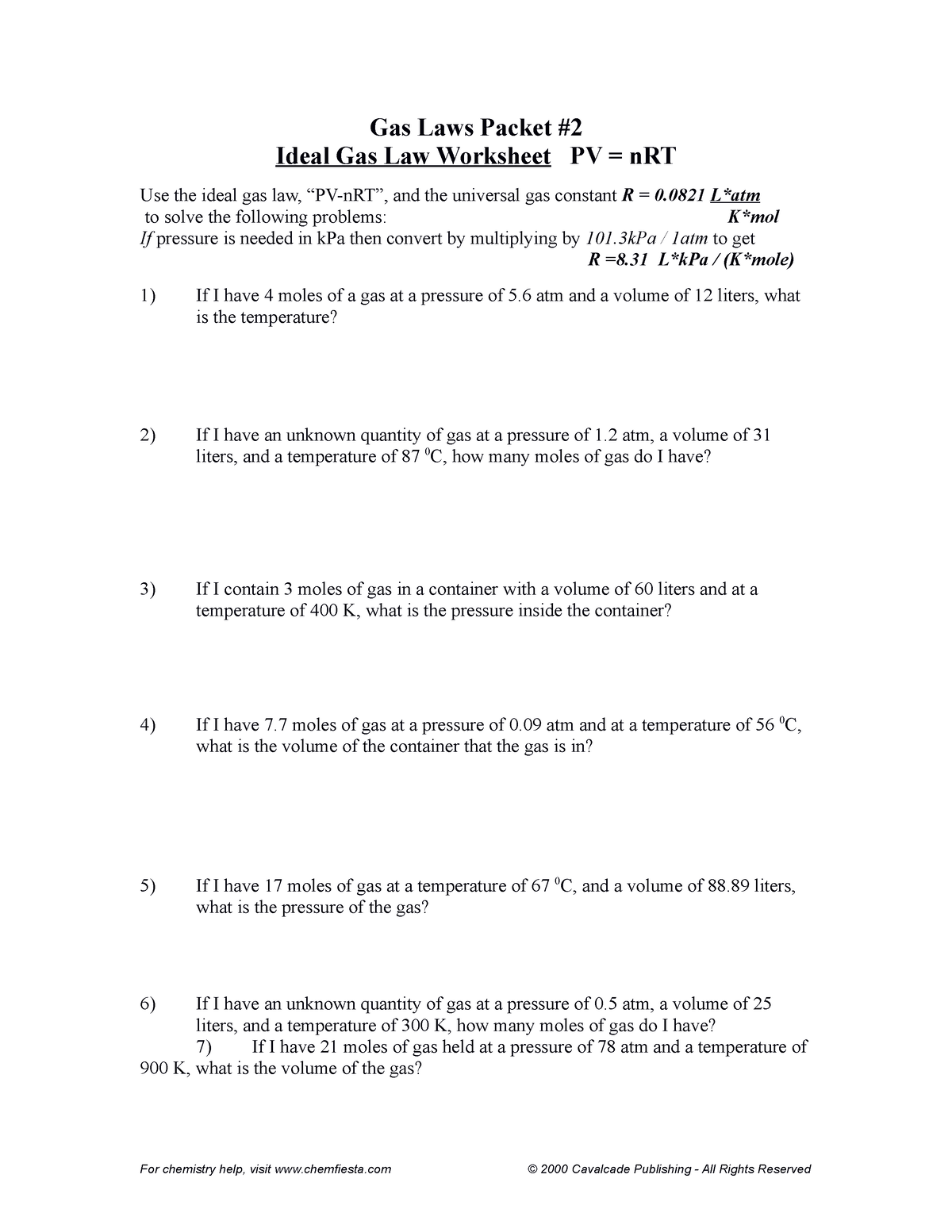
Image: www.studocu.com
Navigating the Ideal Gas Law Packet: Answer Key and Tips
Now, equipped with these key concepts, let’s tackle those practice problems. Imagine a typical Ideal Gas Law practice packet containing a variety of questions, each posing a different scenario involving gases. Here’s how to approach them strategically:
-
Identifying the Given Variables: Carefully read each problem and identify the values you are given. For example, you might be given the pressure, volume, and temperature, and asked to calculate the number of moles of gas.
-
Choosing the Right Equation: Decide if you need to use the full Ideal Gas Law equation (PV = nRT) or if one of the simpler laws (Boyle’s, Charles’s, Gay-Lussac’s) is appropriate for the specific scenario.
-
Converting Units: Ensure all units are consistent with the gas constant (R). If not, convert them to the appropriate units (atm, L, K).
-
Substituting and Solving: Substitute the given values into the chosen equation and solve for the unknown variable.
-
Interpreting the Result: Analyze the result in the context of the problem. Does it make sense given the conditions?
Answer Key Illustration
Consider this example: A container holds 2.5 moles of oxygen gas at a pressure of 1.5 atm and a temperature of 298 K. What is the volume of the gas?
- Given Variables: n = 2.5 moles, P = 1.5 atm, T = 298 K.
- Equation: We use the full Ideal Gas Law: PV = nRT
- Converting Units: Units are already consistent.
- Substituting and Solving:
(1.5 atm) V = (2.5 moles) (0.0821 L⋅atm/mol⋅K) (298 K)
V = (2.5 moles 0.0821 L⋅atm/mol⋅K * 298 K) / (1.5 atm)
V = 40.8 L - Interpretation: The volume of the oxygen gas is calculated to be 40.8 L.
Expert Insights and Take-Aways
Mastering the Ideal Gas Law is not just about memorizing formulas. It’s about understanding the fundamental principles that govern the behavior of gases and applying that knowledge to solve real-world problems. Remember these key takeaways:
- The Ideal Gas Law relates pressure, volume, temperature, and the number of moles of gas.
- The individual laws (Boyle’s, Charles’s, Gay-Lussac’s) describe specific relationships within the Ideal Gas Law.
- The Ideal Gas Law has numerous applications in science, engineering, and daily life.
- Practice is key to mastering the Ideal Gas Law. Work through practice problems and carefully analyze the results.
Ideal Gas Law Packet Answer Key
Embracing the Journey: Your Gas Law Expertise Awaits
The Ideal Gas Law, with its interconnected concepts and diverse applications, is a gateway to a deeper understanding of the physical world. By mastering this essential principle, you’re not just completing a homework assignment – you’re unlocking a powerful tool for analyzing and interpreting the behavior of gases around you. So, grab your Ideal Gas Law packet, dive into the practice problems, and let your understanding of gases reach new heights!





